DermatoConnect
A close relationship exists between sports and psoriasis, sport activity is correlated with the severity and course of psoriasis.1
Here are a few highlights on the relation between physical activity and incidence of psoriasis based on three aspects:
· The Effect of Sports on Psoriasis
· The Impact of Psoriasis on Sport Activities
· The Importance of Sports in Psoriasis
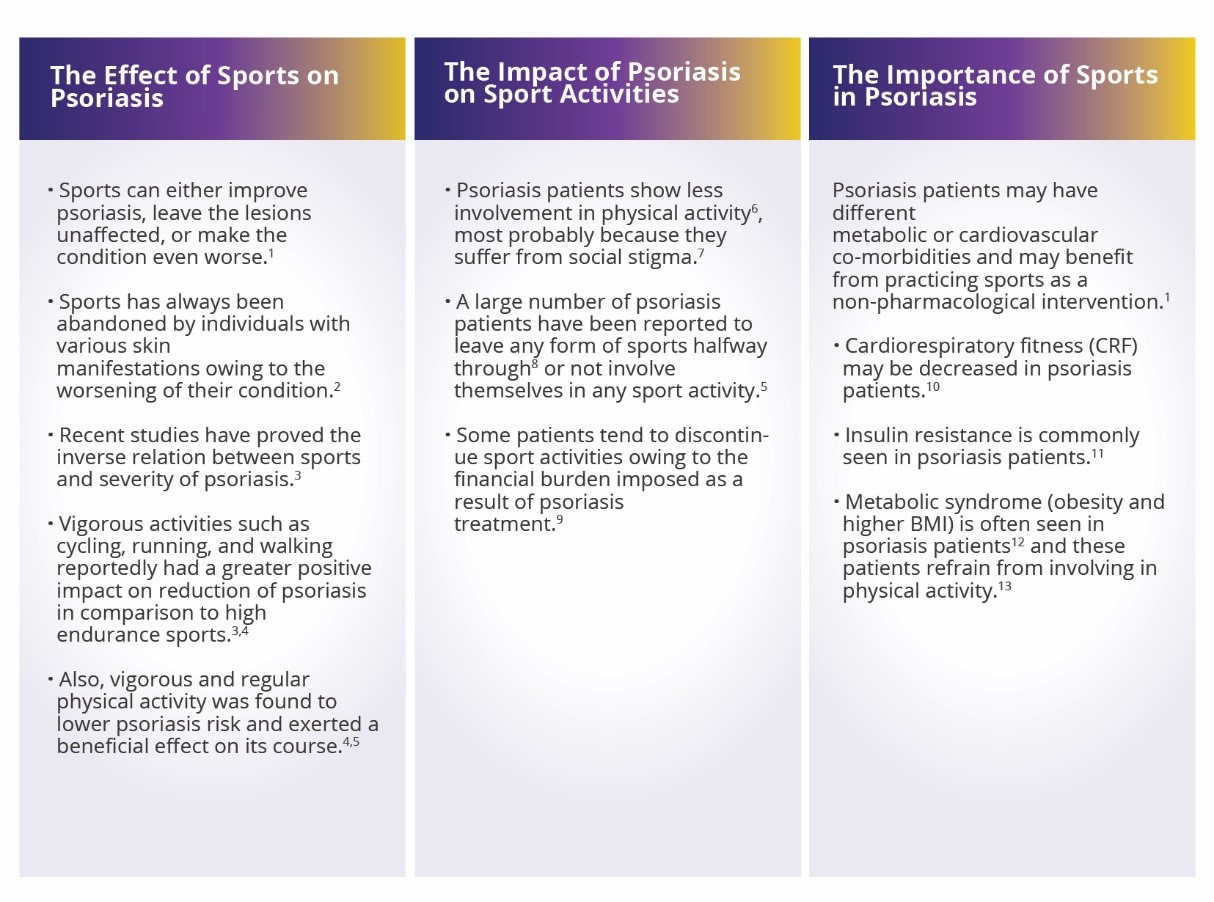
Sports may improve the quality of life of patients with psoriasis. Sport activities seem to be a notable, non-pharmacological source for promoting a healthier lifestyle in psoriasis patients. Yet, the impact of different types of sport activities on psoriasis needs further research.
Source:
1. Custurone P, Macca L, Bertino L, Di Mauro D, Trimarchi F, Vaccaro M, et al. Mutual Influence of Psoriasis and Sport. Medicina
(Kaunas). 2021;57(2):161. 2. Do YK, Lakhani N, Malhotra R, Halstater B, Theng C, Østbye T. Association between psoriasis and leisuretime physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42(2):148-53. 3. Schwarz
PEH, Pinter A, Melzer N, Barteczek P, Reinhardt M. ERAPSO: Revealing the High Burden of Obesity in German Psoriasis Patients.
Dermatol Ther (Heidelb). 2019;9(3):579-87. 4. Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk
of incident psoriasis. Arch Dermatol. 2012;148(8):918-24. 5. Balato N, Megna M, Palmisano F, Patruno C, Napolitano M, Scalvenzi M, et
al. Psoriasis and sport: a new ally? J Eur Acad Dermatol Venereol. 2015;29(3):515-20. 6. Torres T, Alexandre JM, Mendonça D,
Vasconcelos C, Silva BM, Selores M. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study.
Am J Clin Dermatol. 2014;15(2):129-35. 7. Nyunt WW, Low WY, Ismail R, Sockalingam S, Min AK. Determinants of health-related quality
of life in psoriasis patients in Malaysia. Asia PacJ Public Health. 2015;27(2):15. 8. Leino M, Mustonen A, Mattila K, Koulu L, Tuominen R.
Perceived impact of psoriasis on leisure-time activities. Eur J Dermatol. 2014;24(2):224-8. 9. Jenner N, Campbell J, Plunkett A, Marks R.
Cost of psoriasis: a study on the morbidity and financial effects of having psoriasis in Australia. Australas J Dermatol. 2002;43(4):255-61.
10. Wilson PB. Cardiorespiratory Fitness Among Individuals With Psoriasis in the General Population. J Phys Act Health. 2016;13(7):771-
5. 11. Gyldenløve M, Storgaard H, Holst JJ, Vilsbøll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol.
2015;72(4):599-605. 12. Kim HN, Han K, Park YG, Lee JH. Metabolic syndrome is associated with an increased risk of psoriasis: A
nationwide population-based study. Metabolism. 2019;99:19-24. 13. Wilson PB. Prevalence of weight loss attempts and behaviors used
by individuals with psoriasis in the United States population. J Dermatolog Treat. 2017;28(6):515-9.
NON-2022-9859-N














