DermatoConnect
Updates on Atopic Dermatitis and its Management
Atopic dermatitis (AD) is one of the most frequently encountered chronic inflammatory skin condition in the UK.1,2 It affects both, children and adults; however, the paediatric population is predominantly affected.3,4 The overall prevalence of diagnosed AD in European paediatric population was found to be the lowest in Germany (8.4%), while the highest prevalence was observed in the Southern European countries of Spain (18.6%) and Italy (17.6%). Figure 1 depicts the prevalence of AD in children, across different regions of the globe.5 The Global Burden of Disease 2017 data highlights that this chronic condition is highly prevalent in Asian countries, particularly in the high-income territories.6
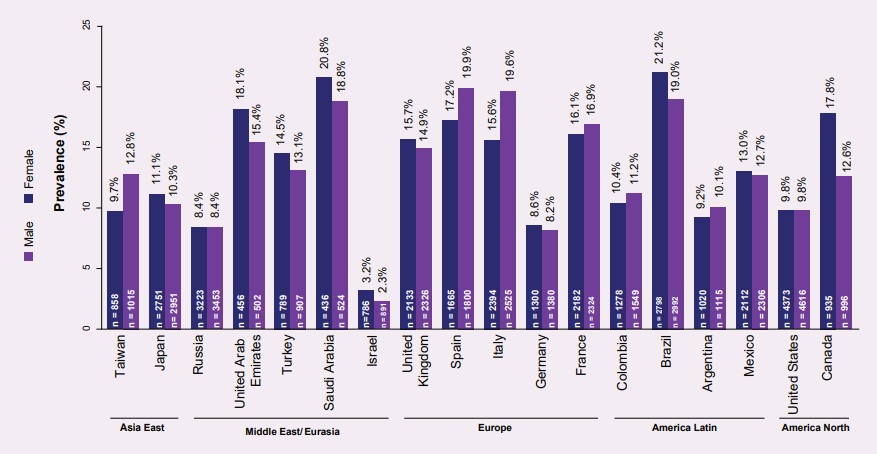
The burden of this disease is substantially high7 and the quality of life of AD patients may be significantly reduced.7,8 Several studies have highlighted the need for psychological support for patients with AD as well as their caregivers to help them cope with the disturbed sleep, disruption of daily life activities, depression, anxiety, and difficulty in maintaining social life.7,8 Also, AD patients are always prone to infections owing to the defective skin barrier, dysregulated immune system, and altered skin microbiome, which may cause complications in these patients.9-11 Health care utilization is significantly increased in AD patients, which also results in significant financial burden.12 Recent developments have promulgated the role of keratinocytes, as a component of the innate and adaptive immune system, in regulating the release of several key molecules triggering inflammatory reactions and immune responses in AD. A better understanding of the pathogenesis would help formulate appropriate treatments to improve the quality of life in these patients and to prevent allergic disorders associated with AD.13
AD has been found to be genetically inheritable.14,15 AD patients express distinct immune and barrier signs, as detected by RNA-sequencing tape strip profiling, which is a minimally invasive technique. Recent studies propose the use of this technique as an alternative to the regular biopsies for detection of AD (both lesional and non-lesional) biomarkers, although further studies are warranted to establish this fact.16,17 With the development of more targeted therapies, AD biomarkers are valuable sources to understand patient-specific molecular dysregulations differing between the several AD subtypes,17 contributing to more tailored treatment and may help to predict which patients are most likely to benefit from the specific targeted therapies.18
A short time ago, treatment for AD mainly targeted emollients, topical steroids, and topical calcineurin inhibitors. Patients who were severely affected by the disease were encouraged to take systemic immunosuppressants. Novel therapeutic strategies, including biologics, aiming at different molecular events involved in the development of AD, have been developed recently.19,20 These options mainly target the type 2 immune pathway and have been found to control the disease effectively and are well-tolerated.1,2,21 The emerging systemic therapies include monoclonal antibodies targeting IL-4,22 IL-13,23 IL-31,24 IL-33, thymic stromal lymphopoietin,9,25 and OX40.26 Latest advances on some probiotic preparations have been shown to be beneficial in decreasing allergic symptoms of AD in children; yet further studies with larger sample sizes are warranted to elude the robustness of this evidence.27 Treatment of a pregnant patient for AD, using systemic drugs, may pose a challenge as it can affect the unborn child. Recent studies advocate that treatment with topical agents should be considered in pregnancy.28,29 The European task force on atopic dermatitis recommends the use of UV radiations in addition to topical corticosteroids. It also adds that moderate sun exposure may be used in such patients.29 Although there have been many promising treatment options for AD, which particularly affects the high-income countries of Asia, further planned prospective studies involving the long-term follow-up of AD patients and cost-effective analyses are warranted to aid clinical decisions in the application of these novel drugs for its treatment.
Source:
1. Plant A, Ardern-Jones MR. Clin Med (Lond). 2021;21(3):177-81. 2. Cork MJ, et al. Journal of Dermatological Treatment. 2020;31(8):801-9. 3. NHS.Available from: https://www.nhs.uk/conditions/atopic-eczema/. 4. Kowalska-Olędzka E, etal. Journal of Drug Assessment. 2019;8(1):126-8. 5. Silverberg JI, et al. Ann Allergy Asthma Immunol. 2021;126(4):417-28.e2. 6. Urban K, et al. JAAD International. 2021;2:40-50. 7. Ražnatović Ðurović M, et al. Ital J Dermatol Venerol. 2021;156(1):29-35. 8. Capozza K, et al. Dermatitis. 2020;31(3):223-7. 9. Wang V, et al. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2021;126(1):3-12. 10. Ong PY,Leung DY. Clin Rev Allergy Immunol. 2016;51(3):329-37. 11. Cai SC, et al. Br J Dermatol. 2012;166(1):200-3. 12. Sandhu JK, et al. Pediatr Dermatol. 2019;36(3):303-10. 13. Chieosilapatham P, et al. Clin Exp Immunol. 2021;204(3):296-309. 14. Brown SJ. J InvestDermatol. 2021;141(1):19-22. 15. Mucha S, et al. Journal of Allergy and Clinical Immunology. 2020;145(4):1208-18. 16. He H, et al. J Allergy Clin Immunol. 2021;147(1):199-212. 17. Renert-Yuval Y, Thyssen, J. P., Bissonnette,R., Bieber, T., Kabashima, K., Hijnen, D., & Guttman-Yassky, E. Journal of Allergy and Clinical Immunology. 2021;147(4):1174–90.e1. 18. Bakker DS, et al. J Allergy Clin Immunol. 2021;147(1):189-98. 19. Ramamoorthy R. Journal of Skin and Sexually Transmitted Diseases.0. 20. Katoh N. J Dermatol. 2021;48(2):152-7. 21. Puar N, et al. Ann Allergy Asthma Immunol. 2021;126(1):21-31. 22. Hajar T, et al. An Bras Dermatol. 2018;93(1):104-7. 23. Furue K, et al. Immunology. 2019;158(4):281-6. 24. Ruzicka T, et al. N Engl J Med. 2017;376(9):826-35. 25. Newsom M, et al. Drugs. 2020;80(11):1041-52. 26. Guttman-Yassky E, et al. J Allergy Clin Immunol. 2019;144(2):482-93.e7. 27. Tan-Lim CSC, et al. Pediatr Allergy Immunol. 2021;32(1):124-36. 28. Napolitano M, et al. Dermatol Ther. 2021;34(1):e14475. 29. Vestergaard C, et al. Journal of the European Academy of Dermatology and Venereology. 2019;33(9):1644-59.
NON-2022-9857-N














